
PDF) Guidance for Industry E6 Good Clinical Practice: Consolidated Guidance | Ataah T - Academia.edu
The EMWA Budapest Working Group: A 2-year collaboration to make recommendations for aligning the ICH E3 guideline with current p
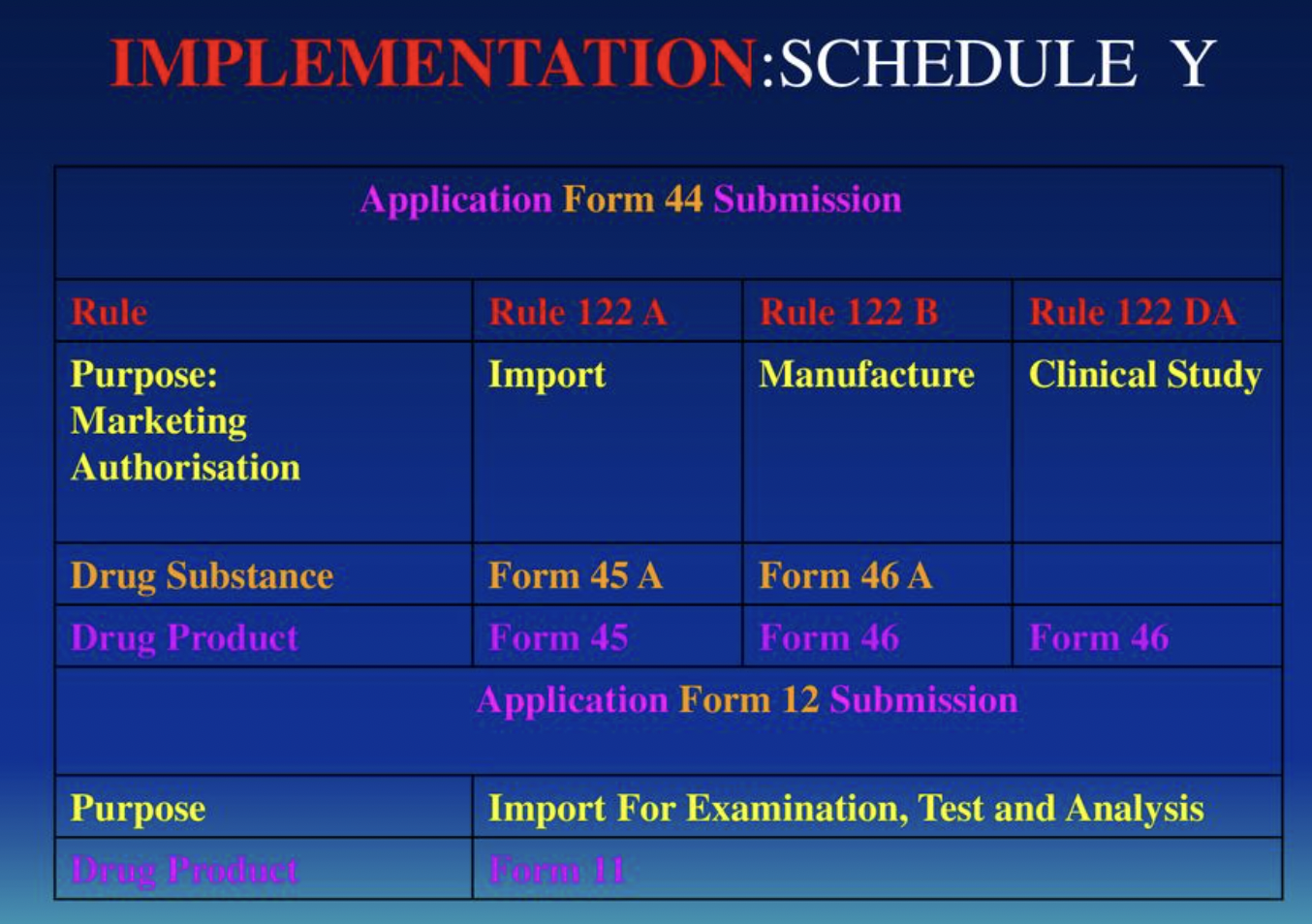
difference between ich gcp indian gcp and schedule y ppt — Clinical Research Certification I Blog - CCRPS

All About Clinical Research : Word Search and Flash Cards for Ich Guidelines for Good Clinical Practice: (Travel Size 2Nd Edition) a Study Guide for the International Council for Harmonisation of Technical

Book M1: 2022 Mini Pocket-Sized (3" x 5") ICH Guidelines for GCP (E6) – Clinical Research Resources, LLC















