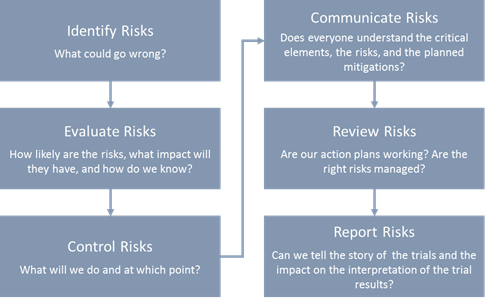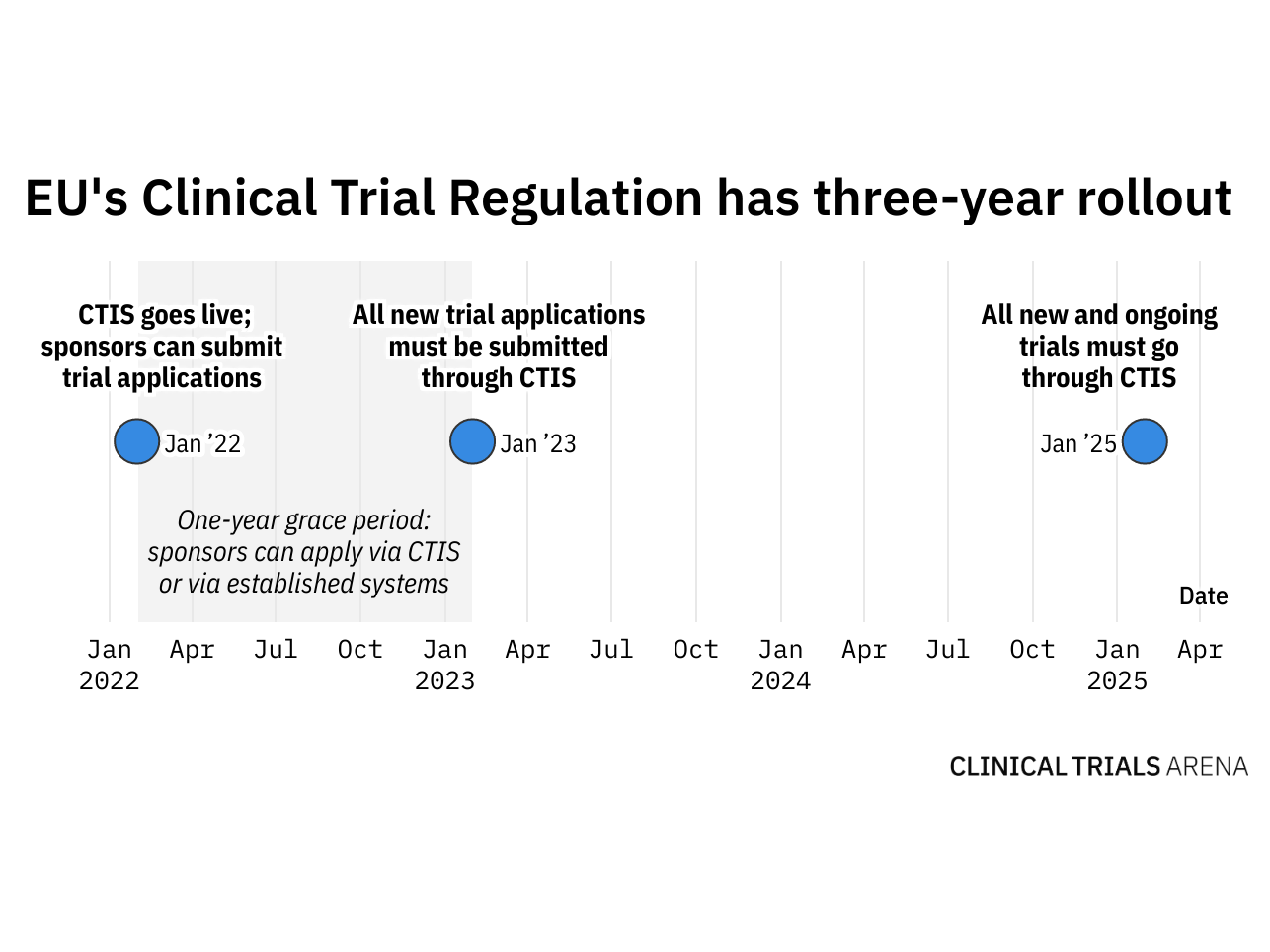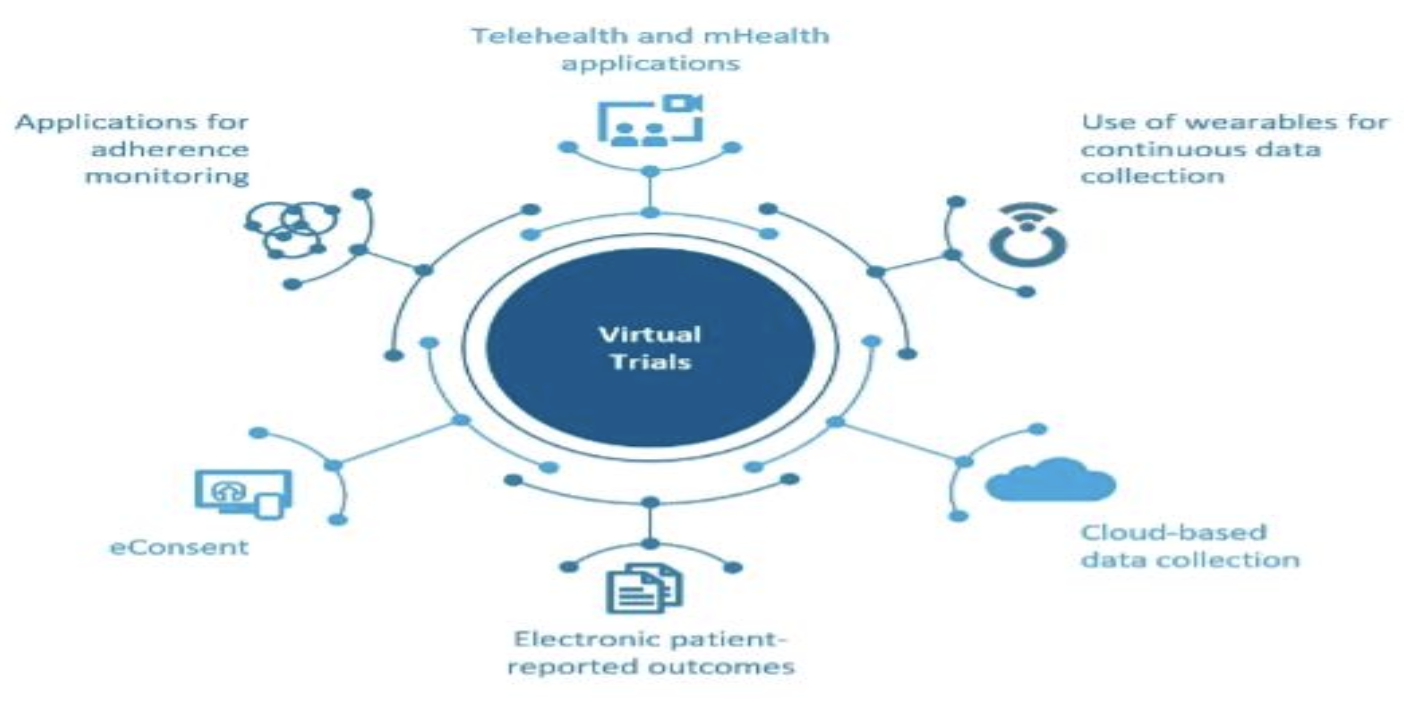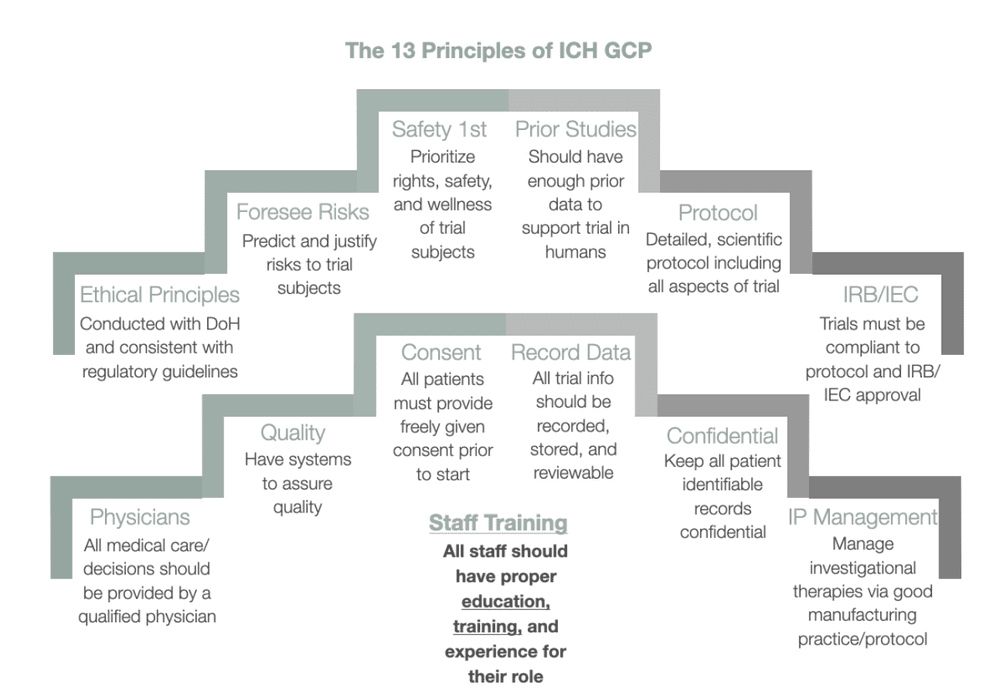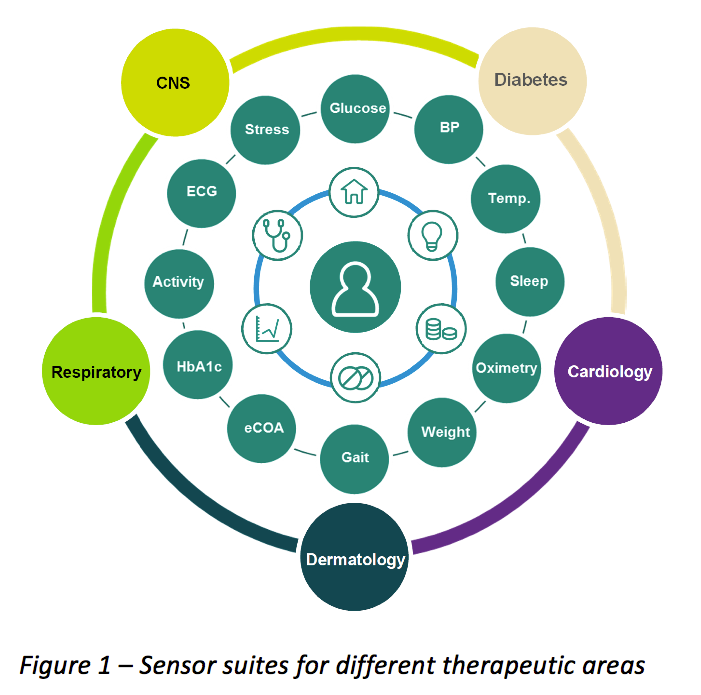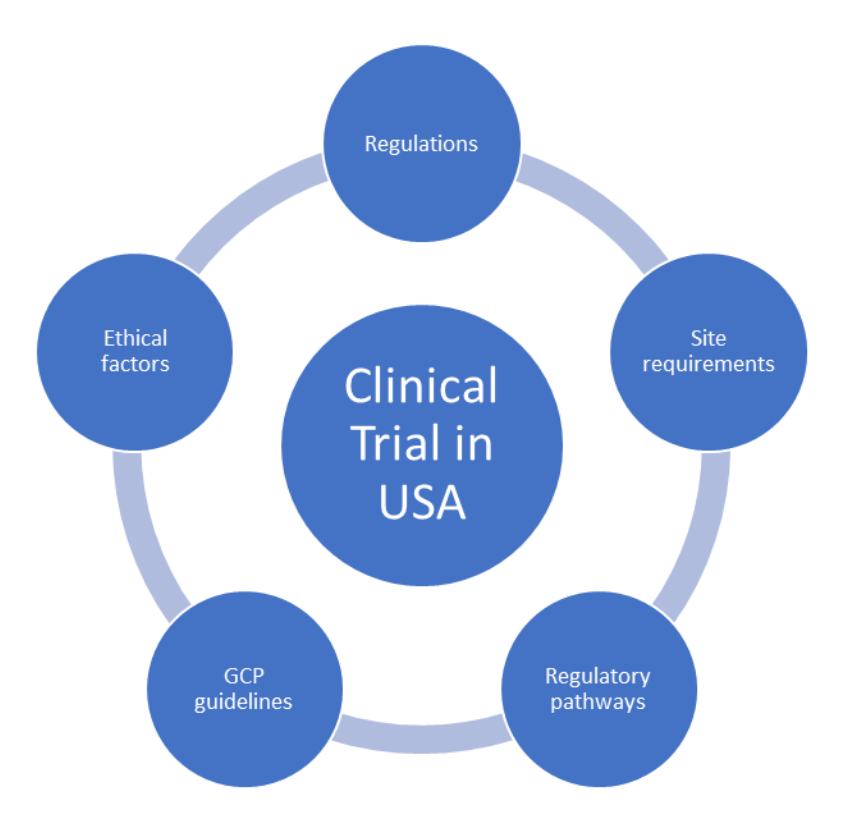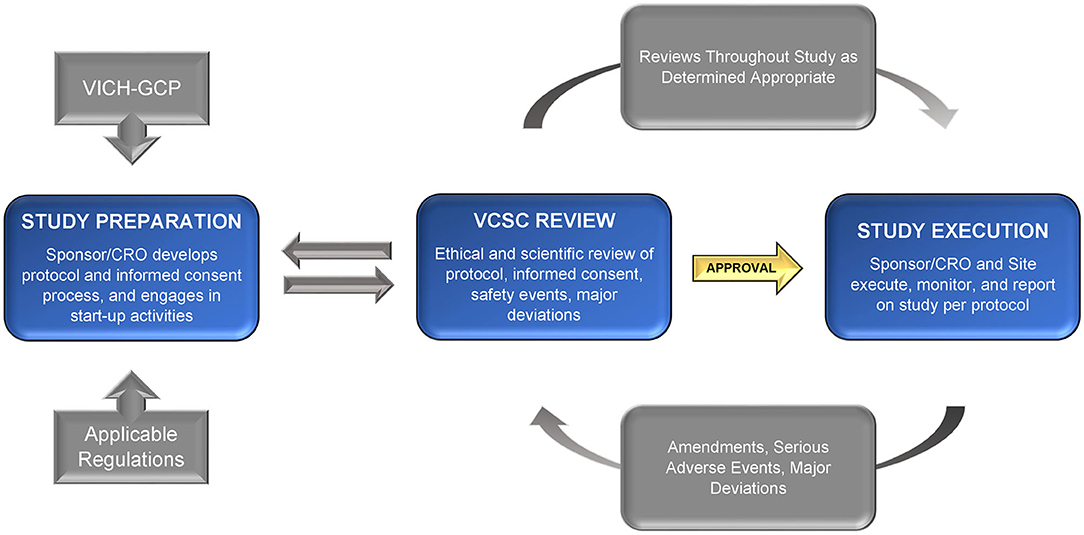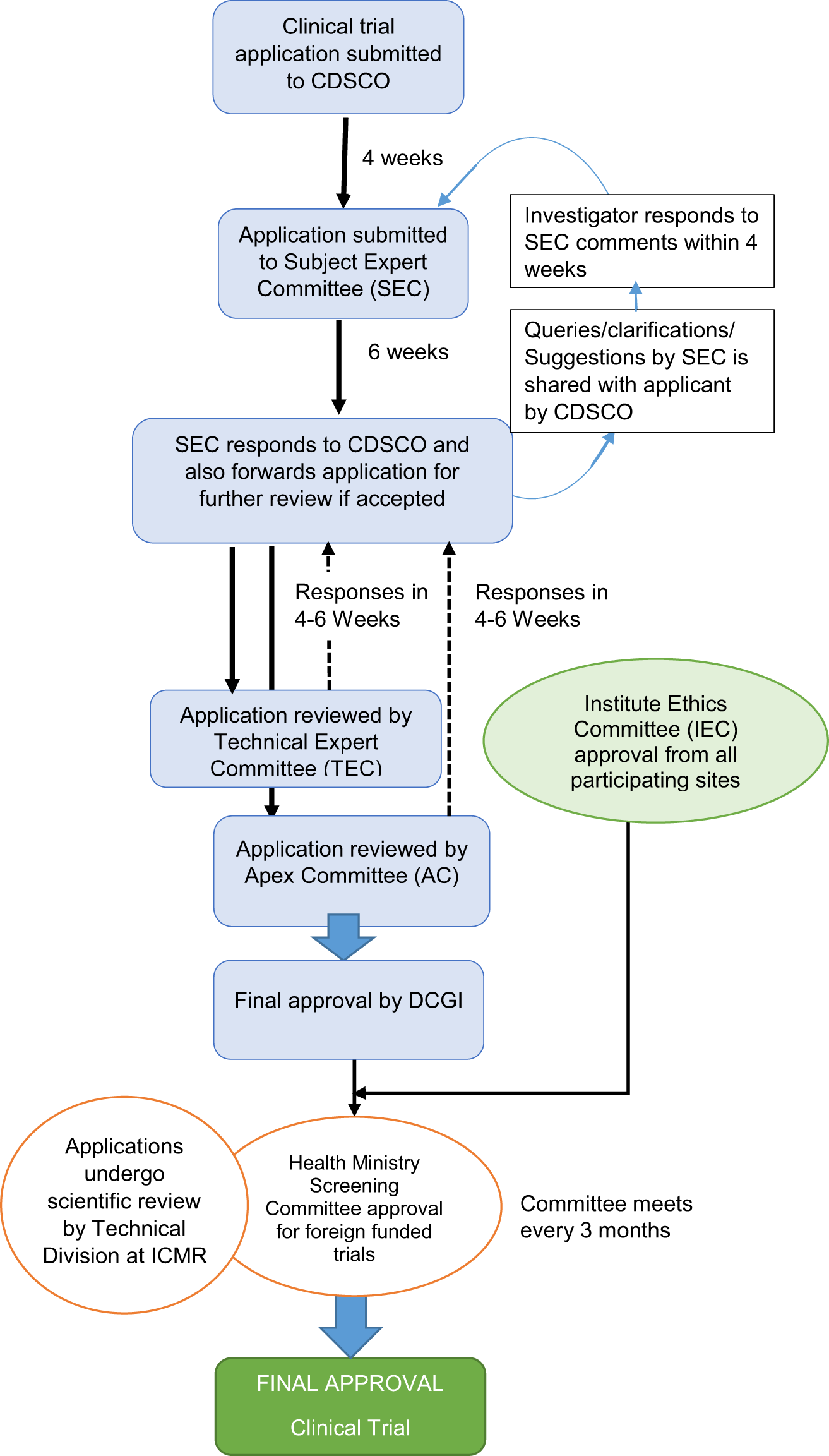
Issues, challenges, and the way forward in conducting clinical trials among neonates: investigators' perspective | Journal of Perinatology

roles and responsibilities of clinical trial personnel — Clinical Research Certification I Blog - CCRPS

Fundamentals of GCP and Clinical Research: 9788192227726: Medicine & Health Science Books @ Amazon.com