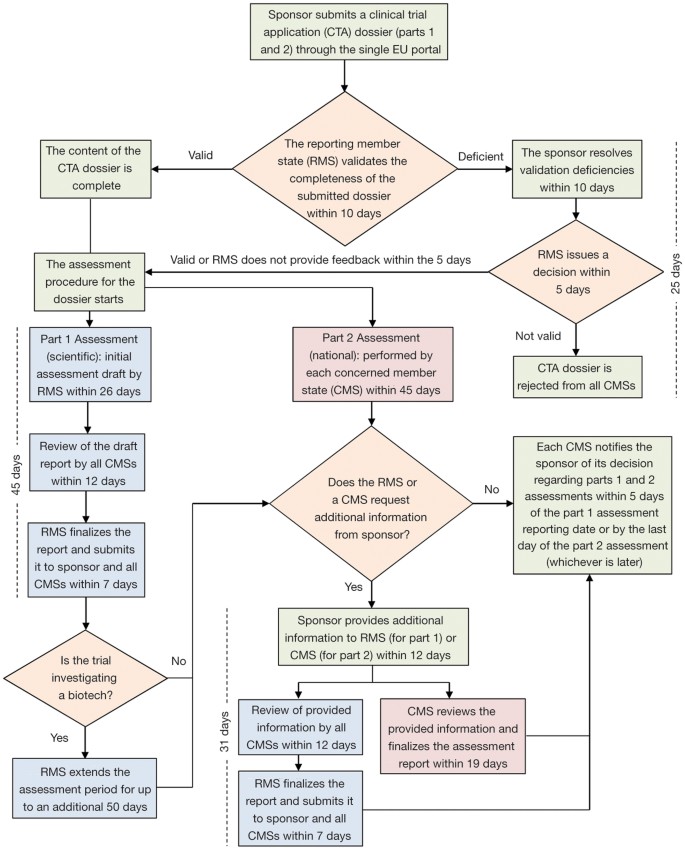
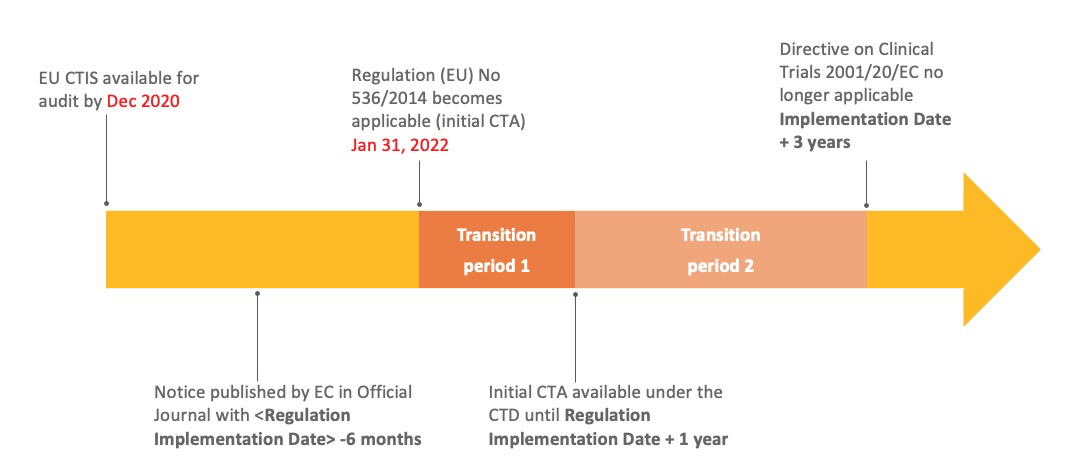
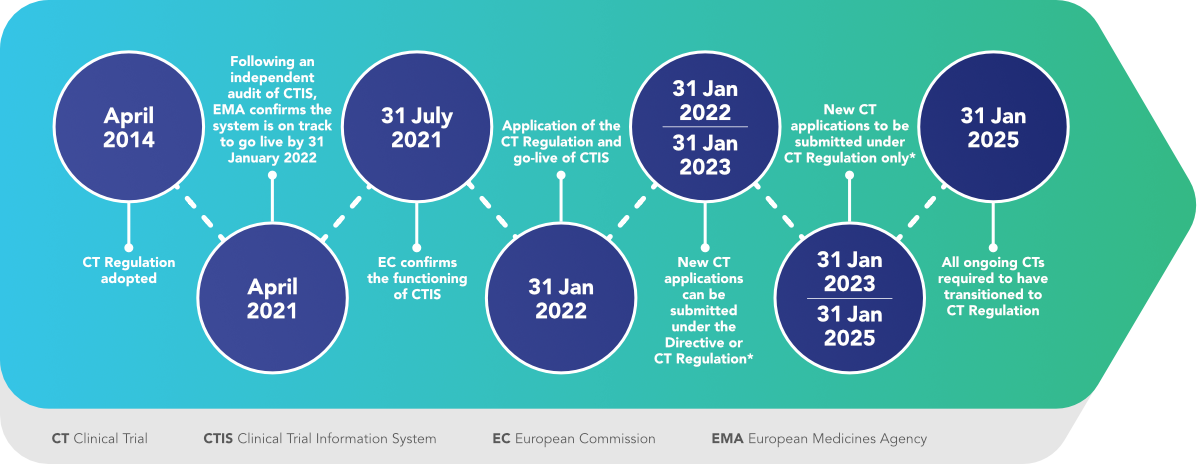
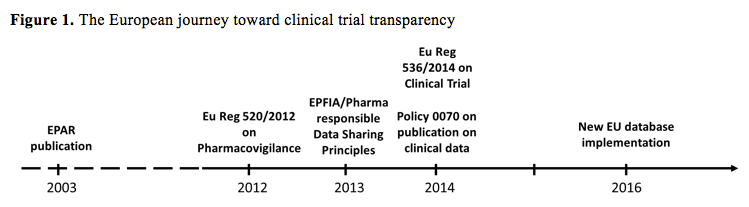
EU Clinical Trial Regulation 536/2014potential timeline. EoT end of... | Download Scientific Diagram

Solve Expiry Labels, DtP, and Timelines for EU 536/2014 Clinical Trials Regulation | Healthcare Packaging

Managing the New EU Clinical Trials Regulation 536/2014 – Guidance for Navigating the Clinical Trial Information System (CTIS)