Recruitment of Black Adults into Cardiovascular Disease Trials | Journal of the American Heart Association

PPT – Clinical trial protocol writing: Challenges and Guidelines PowerPoint presentation | free to download - id: 906c6c-ODNhN
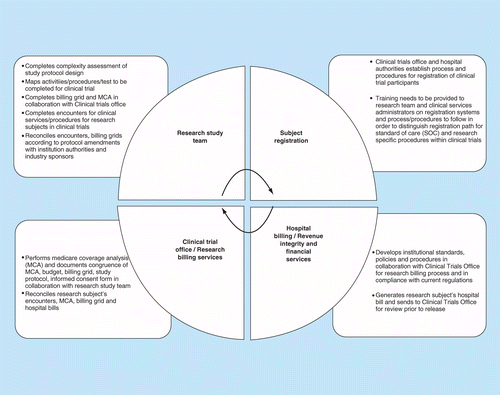
Optimization of protocol design: a path to efficient, lower cost clinical trial execution | Future Science OA
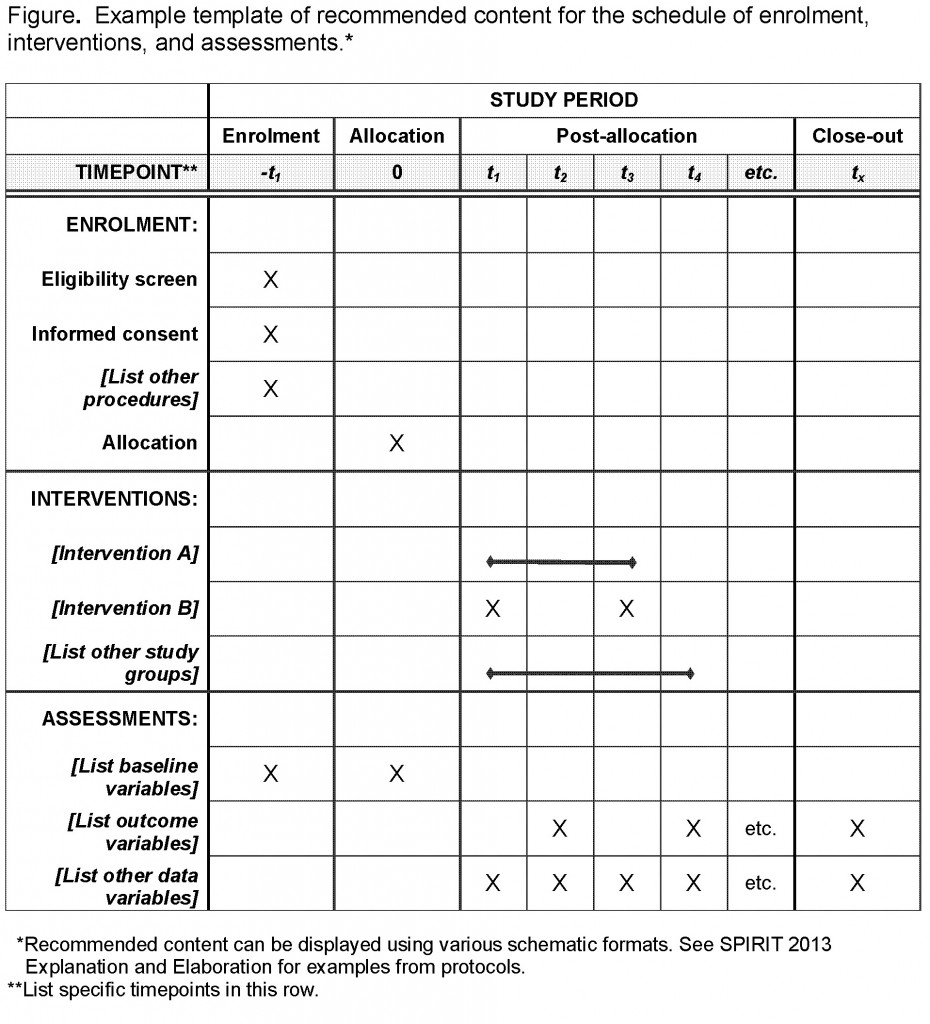
![PDF] SPIRIT 2013 statement: defining standard protocol items for clinical trials. | Semantic Scholar PDF] SPIRIT 2013 statement: defining standard protocol items for clinical trials. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/ccf1230c8d03a35229dc9fd36776d69c3356cfa3/2-Table1-1.png)
PDF] SPIRIT 2013 statement: defining standard protocol items for clinical trials. | Semantic Scholar

Assessing the detection, reporting and investigation of adverse events in clinical trial protocols implemented in Cameroon: a documentary review of clinical trial protocols – topic of research paper in Clinical medicine. Download

Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension - The Lancet Digital Health

Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension - The Lancet Digital Health
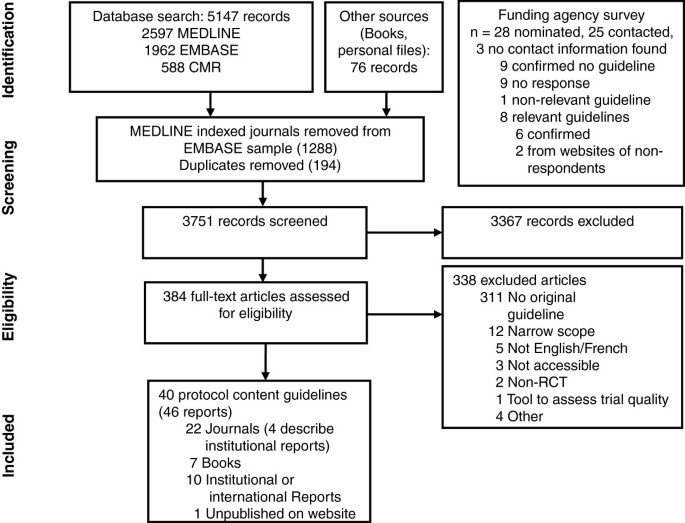
Guidelines for randomized clinical trial protocol content: a systematic review | Systematic Reviews | Full Text